The art of creating artificial cells
An interview with Petra Dittrich, Professor for Bioanalytics at ETH Zurich.
About the smartness of cells, the challenge of copying them and the crucial impact of an ERC Grant on the career of a scientist.
Engineering of hybrid cells. How do you explain to your 11-year-old son what you are doing in this project financed by an ERC Grant?
I would say: Look at your body. It consists of millions of tiny cells. Your skin, your heart, your blood, all is made of cells. Cells are the smallest units of life. They live. They can produce molecules, eat molecules, change molecules and use molecules for their own purpose. With our project we want to learn how cells can do all these fascinating things and at the same time we would like to copy them. We wish to build artificial cells by ourselves that have at least some of the amazing capabilities of natural cells. But in order to build artificial cells, we need specific molecules that natural cells also consist of. These molecules are called lipids. We use them to form a small vessel shaped like a little ball or a balloon. Into this ball, we pack everything a cell needs to perform certain activities. The shell of the ball, a tiny membrane of lipids, is strong enough to keep all the cell molecules inside. So, the aim of our project is to learn how to build such artificial cells and how to make them work for us.
How do you manage to build such artificial cells?
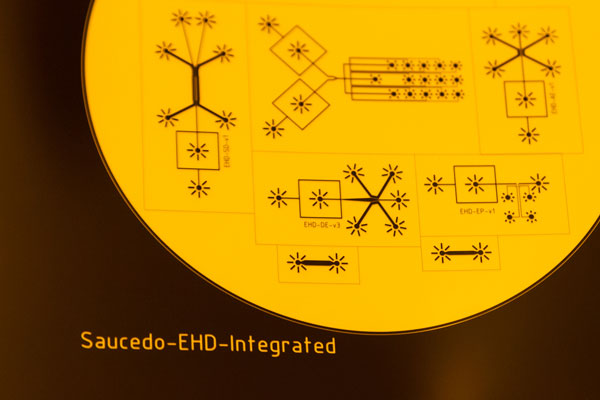
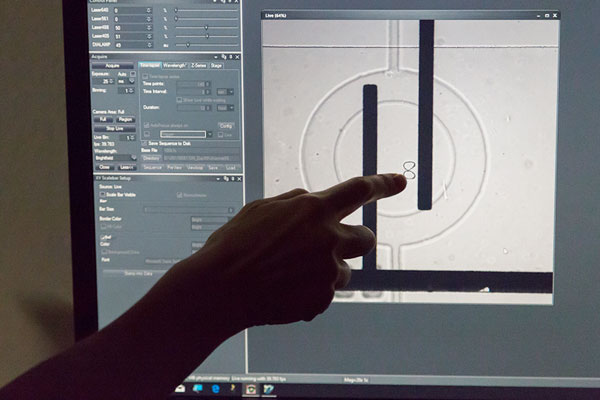
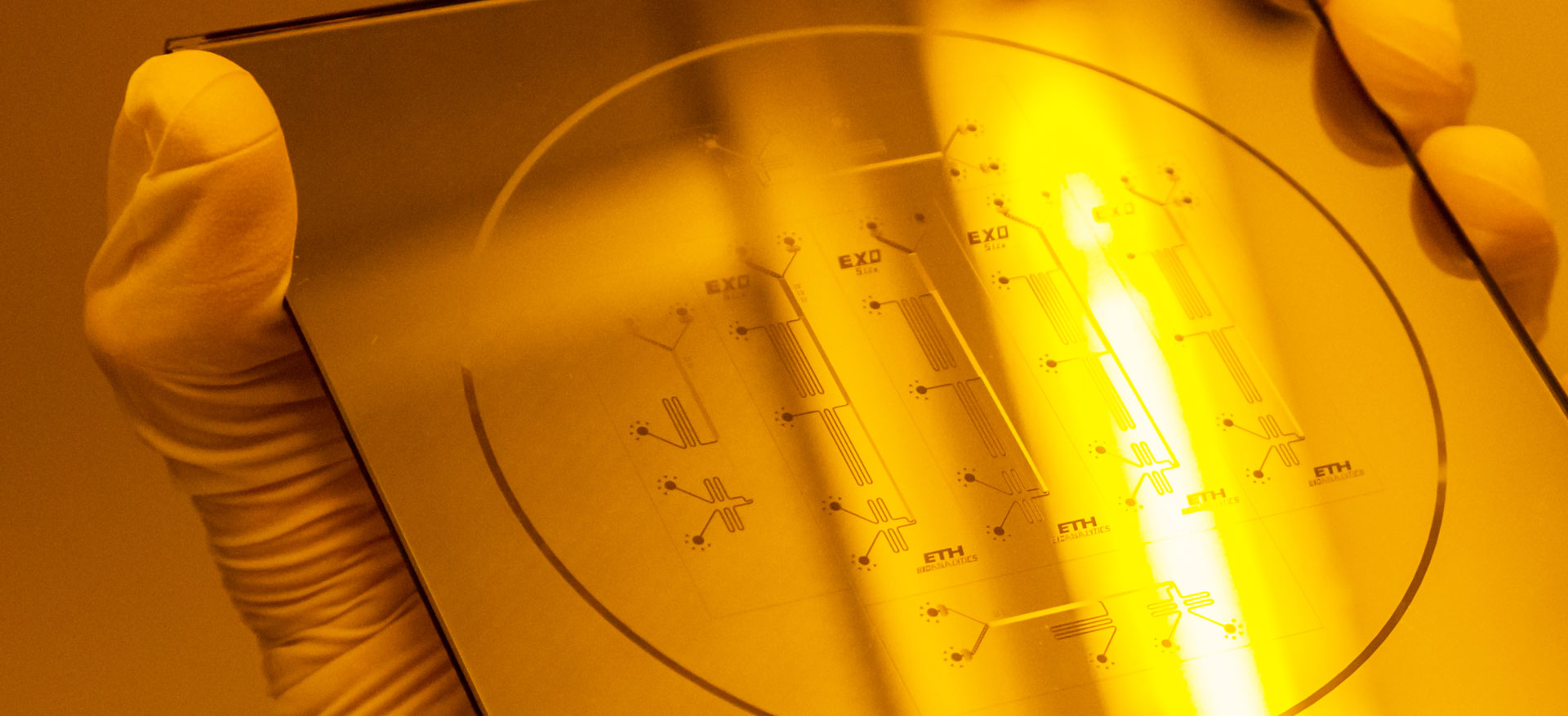
We fuse artificially produced lipids and lipids from a living cell. The result is a hybrid cell, partly artificial and partly natural. The production of such cells, however, is a big challenge. You can bring lipid molecules together, but they will not form a ball just by themselves. Here, our technology comes in. It is called lab-on-a-chip technology and this is the second aspect of our project. We develop little devices or «microchips», which enable us to form these little lipid balls and produce artificial cells. The devices also help us to position the cells, to monitor them, to watch them under the microscope to see what is happening.
What will you do with these artificial hybrid cells?
Like our natural cells they can produce molecules, they communicate with each other and they can detect and identify molecules. So, we try to stimulate biochemical reactions within the hybrid cells similar to processes that would also take place in a natural cell. They work like a bioreactor producing peptides and proteins very efficiently.
And what is the benefit of hybrid cells compared to natural cells?
They work for us, producing or detecting molecules, but they do not live. They do not grow, do not divide and do not reproduce. We only add those elements from living cells into the hybrid cells that are required to get the expected result. We don’t want to create artificial life. As we focus on one specific reaction or process, there are no or not many side reactions and consequently, we can observe the selected aspects in a much clearer way.
What is the aim of your ERC project?
The overall goal is to invent methods that are generally useful for the construction of artificial hybrid cells in a controlled way for producing or sensing molecules. At the same time, we learn how cells are doing just that and how the compartments in the cells cooperate. We find out which ones of the components are required; for example, which type of lipid is essential. To achieve these goals, we constantly have to adapt and develop our lab-on-a-chip devices and biochemical methods.
Which practical applications could the results of the project produce?
One application are biosensors, which are extremely sensitive to certain molecules. They work like a natural cell, where a molecule –the ligand – binds to a receptor protein in the membrane. Our sensors are not alive. They can be inserted into little instruments, e.g. little diagnostic chips, to detect biomarkers and thereby collect information from inside our body.
«We wish to build artificial cells
that have at least some of the amazing
capabilities of natural cells.»
They can also be used for pharmaceutical or medical applications like drug screening. The current methods are commonly based on cells or cell cultures. Replacing natural cells by artificial hybrid cell systems would have many benefits in terms of reduction of laborious work, reliability and reproducibility.
And what are the main challenges you are facing?
Our lab-on-a-chip devices are very established and we are really good and experienced in building them. Now we are facing the challenge that cells are very complex. We can already fabricate simple models, but it is indeed very demanding to make them more sophisticated, more like a natural cell. It is stunning how a living cell organises its architecture on its own. We have to work hard for it to create a simplified model.
What fascinates you so much about your project?
Its interdisciplinarity between cell biology and engineering. Engineering is very result-oriented, we achieve the goals by inventing the required technology. But it is also very creative to design and build the lab-on-a-chip devices. And then it is great to see that they work. These new methods based on lab-on-a-chip devices are unique and help us to increasingly understand how cells operate and how they behave in different environments.
Let us talk about the ERC Grant again. What motivated you to apply for a Consolidator Grant?
I already received an ERC Starting Grant in the very first call and greatly enjoyed the freedom I had thanks to this funding. As I believed that my new project is important, has great potential for the future and will have an impact on different fields I was convinced that I had fair chances to receive a Consolidator Grant. Therefore, I applied. It was also the right moment for me regarding the stage I had reached in my career. I applied for the ERC Starting Grant just before I became assistant professor. After the step to associate professor, I applied for this Consolidator Grant and received it.
For a researcher, what are the main benefits of an ERC Grant?
In a nutshell: independency and funding over a long period of five years. When I applied for the Starting Grant, I was a postdoc embedded in a larger group. But I really wanted to prove to myself that I could do research independently and that I was able to do this without any supervisors. I strongly wanted to realise my own ideas and also supervise students and PhD students. So, I think an ERC Starting Grant really gives you the freedom to try out things but also to prove yourself at an early stage.
«It is stunning how a living cell
organises its architecture on its own.»
I was already independent when I received the ERC Consolidator Grant. There, I need it because it gives me the freedom to address new topics and move in a certain direction. I can explore this direction for the next few years and, together with my team, concentrate specifically on this project for some time. And of course, it is also very prestigious.
Did you get any help when you applied?
The support from the EU GrantsAccess office was extremely helpful as they are very experienced and know exactly what must be considered. For example, I appreciated the help to set up the budget. I was insecure, say, about whether I should rather apply for postdocs or PhD students and they helped me to find the right answers.
Would you recommend applying for an ERC Grant?
Absolutely.
Even though it requires much bureaucracy?
Oh no, there is not too much of bureaucracy. Considering the size of the grant you receive, the amount of financial and scientific reports you have to write is quite reasonable. And you always receive support by the EU GrantsAccess office. It is clearly the best funding scheme you can get at the moment.
You are a professor at BSSE based in Basel. How is the scientific environment there?
The BSSE hosts research groups with background in experimental biology, bioinformatics and engineering. It is very inspiring to work in an institution where you have all these different disciplines under one roof. We have for example a «science lounge», where we can meet for exchange and discussions. Interdisciplinarity sounds great, but it is indeed challenging. It takes time to understand the concepts and the language of the other disciplines. It is unusual that you sit together, immediately come up with the greatest ideas and you have a success story the week after. It is a process, for me and particularly for new team members, which takes a while. But it is extremely enriching. For instance, we had very good discussions here with microbiologic groups and that inspired me to increase efforts in this direction and made me realise the importance of this field. Moreover, I like the Basel area because we are also near the University of Basel and private companies who are engaged in engineering and method development.
What fascinates you so much about cells and bioanalytics?
I am a chemist by education, but at some point in my studies I realised that I am not the person to design and synthesise molecules. In organic chemistry you need these big devices and equipment to produce molecules. You have to purify the molecules again and again and go to the next synthesis steps and so on. I did not like that very much. But during my PhD time in a biophysics group, I realised that cells can produce molecules very smartly without these big apparatuses.
«We don’t want to create
artificial life.»
They don’t have to purify the molecules in a process of many steps, they simply produce them in a very efficient way, while deviations from these biochemical pathways are the origin of diseases. This complexity in dimensions of a few micrometres fascinates me.
Life Science is a very dynamic field. How will it change our life in the years to come?
There is great progress in the fields of biosensors, implanted sensors, sensors in mobile devices like your cell phone. I think in the near future we will use these sensors for new types of diagnostic instruments in a much easier way than nowadays.
«We want to be at the forefront,
being the first to show
that it works.»
Constantly monitoring health and therapies is definitely a trend that is also related to our field. Another trend is what we call body-on-a-chip, trying to make functional tissues placed on small-scale devices and to use them to test the efficiency of drugs on a personalised level. You will see immediately if the drug works efficiently for you or how it is metabolised.
Ambitious researchers reach for the stars – what are you reaching for?
We want to leave an important and long-lasting imprint on our field, improve analytics and diagnostics. Regarding the ERC project with the hybrid cells, we want to be at the forefront, being the first to show that it works. And in a next step we wish to make it available to others so that, finally, people can use our hybrid cells.
Engineering of hybrid cells using lab-on-a-chip technology
To learn about the fundamental characteristics of cellular organisation and compartmentalisation, in particular the role of lipid membrane, and to exploit this novel knowledge for engineering minimal cells so that they have both a great impact in the context of synthetical biology and also for pharmaceutical and medical applications.
Lab-on-a-chip
is a little device that integrates one or several laboratory functions on a single chip the size of millimetres to a few square centimetres. Lab-on-a-chip enables to manipulate fluids very precisely in tiny volumes of nanolitres or picolitres which cannot be handled with standard lab equipment like pipets. The devices open the way to novel analytical approaches in many different areas such as the examination of cells, cell cultures or tissues, the manipulation of tissue and for diagnostic purposes like blood analyses.
Interview with Petra Dittrich
Petra Dittrich
Petra Dittrich has been Associate Professor for Bioanalytics at the Department of Biosystems Science and Engineering BSSE of ETH Zurich since 2014. The BSSE Department is located in Basel. Her research in the field of lab-on-a-chip technologies focuses on the miniaturisation of high-sensitivity devices for chemical and biological analyses and microfluidic-aided organisation of materials. She studied chemistry at the Bielefeld University (Germany) and the Universidad de Salamanca (Spain) from 1993 to 1999. In 2003, she received her PhD degree at the Max Planck Institute for Biophysical Chemistry in Göttingen (Germany). After another year as postdoctoral fellow at the Max Planck Institute, she held a postdoctoral position from 2004 to 2008 at the Institute for Analytical Sciences ISAS, Dortmund (Germany). For research stays, she was at the Cornell University in 2002 and the University of Tokyo in 2005. In 2008, Petra Dittrich was nominated Assistant Professor for Bioanalytics at the Laboratory of Organic Chemistry of the Department of Chemistry and Applied Biosciences at ETH Zurich. Petra Dittrich received an ERC Starting Grant in 2008 and an ERC Consolidator Grant in 2016.
ERC Consolidator Grant
«HybCell: Engineering of hybrid cells using lab-on-chip technology»
Duration: 2016-2021
Financial contribution from H2020: 1,971,250 €
Concluded FP7 projects:
ERC Starting Grant «nmu-LIPIDS: Biomimetic Lipid Structures on Nano- and Microfluidic Platforms»
Duration: 2008-2014
Financial contribution from FP7: 1,941,000 €
Collaborative project «ROC: Radiochemistry on Chip»
Duration: 2008-2011
Financial contribution from FP7: 218,314 €
Partners: 6 institutions from 5 different countries